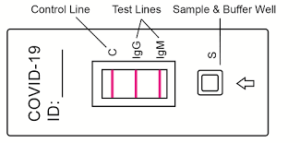
Rapid Antigen tests for COVID 19
Rapid Antigen testing : Detecting COVID 19 virus using a simple rapid test.
*Ngaio is currently applying for authorization to sell or import rapid antigen tests for COVID 19. For those who would like more information and updates, please sign up on our homepage https://shop.ngaio.co.nz or email us info@ngaio.co.nz
What is a RAT? Rapid Antigen Test
A rapid test designed to detect active cases of COVID-19. The tests detect specific proteins in the virus. Sampling is generally done through a nasal swab rather than the deep and uncomfortable nasopharyngeal swabs used in PCR tests.
The New Zealand Ministry of health provides this outline:
“Antigen tests are used for diagnosing an acute, active COVID-19 infection. The main advantage of Rapid Antigen Testing (RAT) is that results can be available as soon as 15 minutes after the sample is taken. They are also relatively cheap to perform”.
A positive antigen test result is considered accurate when instructions are carefully followed. It should be recognized however that sensitivity and specificity does not match laboratory-based methods. A PCR test can be used to confirm a positive or negative antigen test result.
How is a RAT different from other COVID tests?
A PCR test is currently the most accurate test available and is what New Zealand currently uses for laboratory testing. It is more expensive, takes longer and requires specialist equipment and laboratory personnel.
An antibody test shows whether you were infected previously. These should not be used as a screen for current infection but might be of interest for the individual to know. They are often blood tests.
COVID-19 test regulations in New Zealand:
No one is able to import, manufacture, supply, sell, pack or use a rapid COVID-19 test, without approval from the Ministry of Health. The legislation is available here:
https://legislation.govt.nz/regulation/public/2021/0066/latest/whole.html#LMS451450
Rapid antigen testing for businesses and organizations vs self-testing for individuals and families:
Under the existing government legislation – authorization is required to use COVID-19 Antigen rapid tests.
Initial authorizations are therefore likely to be for organizations in NZ with the appropriate systems and training in place: Part of the application for authorization asks the user to provide information on how records of the testing will be managed. There is also a requirement to explain how identification of the person being tested will be done as well as how the date/time of the test and the test result will be recorded. Further there is a requirement to explain how appropriate clinical management of individuals and notification of “non-negative” results to the Medical Officer of Health will occur.
The use of antigen rapid tests for self-testing is unlikely to be available in the near future.
Training and internal testing protocols are likely to be an important factor.
Ngaio Diagnostics can help by assisting with your application for authorization to use RATs, training your staff and establishing systems for COVID rapid antigen testing.
Posted in News | Comments Off on Rapid Antigen tests for COVID 19 */?>